Our Research
Therapeutic Targeting of Dystrophin-Targeting microRNA
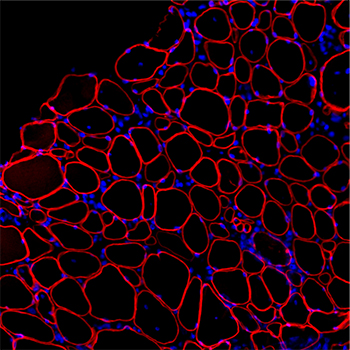
We have found that in dystrophic muscle there are elevated levels of microRNAs that inhibit dystrophin translation. The presence of these microRNAs in muscle may explain why dystrophin rescue after exon skipping therapy shows so much variability. One goal of our laboratory is to inhibit or genetically delete these miRNAs in animal models and determine if this will improve dystrophin rescue. While our initial projects focus on targeting these microRNAs in DMD in combination with exon skipping therapies, we are also exploring their role in other muscle disorders where a secondary dystrophin deficiency could contribute to exacerbating inflammation and muscle damage.
The Role of Inflammatory microRNAs in Duchenne Muscular Dystrophy and Other Inflammatory Muscle Disorders
Genetic Models of Becker Muscular Dystrophy
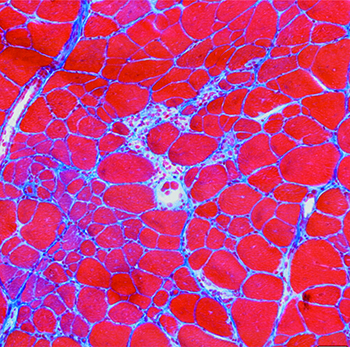
Becker muscular dystrophy (BMD) occurs from in-frame mutations of the dystrophin gene; thus, a decreased amount of truncated dystrophin is produced in the muscle. This disease is not as well researched because it is assumed that symptoms are milder; however, this disease is often quite variable in severity and the muscle function and heart health of these patients is often compromised. Our lab has also observed increased dystrophin-targeting microRNAs in the muscle of patients with BMD.