Our Research
Research in Pediatric Brain Development
The Haydar Laboratory has two major themes, one based on understanding how gene expression and cellular diversity contribute to the complex circuitry and function of the brain and another uncovering the molecular and cellular basis of cognitive dysfunction in developmental disorders, primarily Down syndrome.
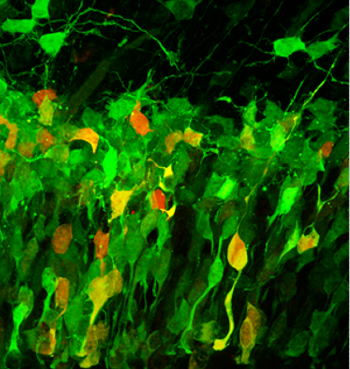
Our studies on basic neocortical development are currently examining the molecular diversity of the stem and progenitor cells that together produce all the neurons and glial cells in the neocortex.Using single cell transcriptomics in a variety of mammalian species and in vivo labeling of discrete progenitor groups, we are learning how gene expression and cell fate decisions eventually culminate in cortical circuitry. Studies in this area include bioinformatics analyses and species comparisons as well as electrophysiology and optogenetic recording of neurons from different progenitor families. Finally, we use cognitive tests in mice to consolidate our findings at the level of complex behaviors.
Our study into the underlying causes of intellectual disability in Down syndrome has uncovered several interesting findings that may lead to improved quality of life and independence for people with Trisomy 21. Using a variety of systems including mouse models, human tissue and induced pluripotent stem cells, we have identified significant changes in neural precursor cells prior to birth and have demonstrated that changes in oligodendrocyte differentiation and maturation may lead to decreases in the amount of myelin in the brain. As myelin, formed by oligodendrocytes, is critical for fast signaling in the brain, our studies are investigating the molecular mechanisms leading to less myelin and are developing cellular platforms to test possible drug treatment avenues.
Methodology
Cognitive Testing
We have a core behavioral suite for testing rodent cognitive and motor behavior which includes various water mazes, open field testing and neonatal milestones analysis.
Confocal/Multiphoton Microscopy
We use several systems optimized for in vivo and in vitro time-lapse imaging studies as well as for 3D scanning of fixed preparations. In addition, a new dual 2-photon optogenetics microscope coupled to a patch-clamp rig enables direct stimulation and recording of neurons at the same time they are being imaged.
Image Analysis
We use several analysis programs to reconstruct and quantify fluorescence image volumes. The capabilities include following cells in four-dimensional time lapse studies, quantifying the numbers and distributions of cells and deconvolution of image stacks to count and classify dendritic spines.
In Utero Electroporation (IUE)
We use IUE to introduce DNA expression vectors into fetal neural precursor cells to determine the role of a particular gene or mutation in brain development. This technique allows us to image and/or quantitatively measure the consequences of gene expression directly in the three-dimensional environment of the developing brain.
Gene Expression Profiling
We are using fluorescence activated cell sorting, droplet capture and RNA sequencing to understand how gene expression patterns influence brain development in both normally developing brains and in brains of humans and mice with Down syndrome.
Immunohistochemistry
We use immunohistochemistry to localize proteins of interest within sections of the developing and mature nervous system. Usually the antibody staining is followed by fluorescence imaging and quantification.
Cell Proliferation Assays
We use various techniques, including immunohistochemistry and in utero electroporation, to measure the proliferation of neural precursor cells both in vivo and in culture.
Electron Microscopy
Ultrastructural studies are conducted to examine subcellular elements of nervous system anatomy, including the establishment and maintenance of myelin.
