Our Research
Gene Therapy Delivery Strategies for Muscle Diseases
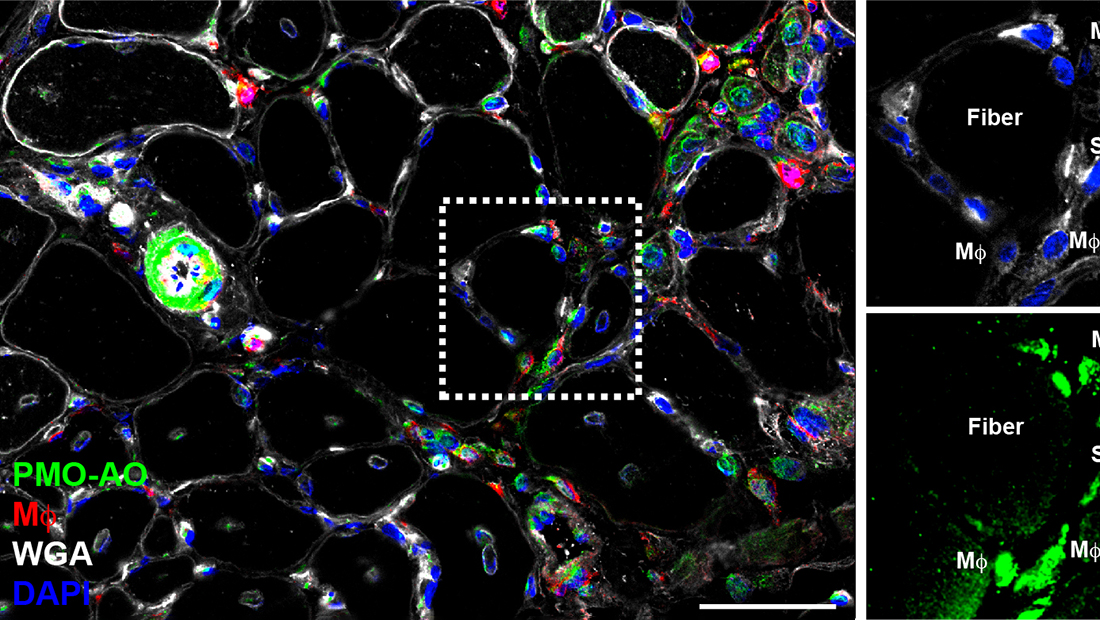
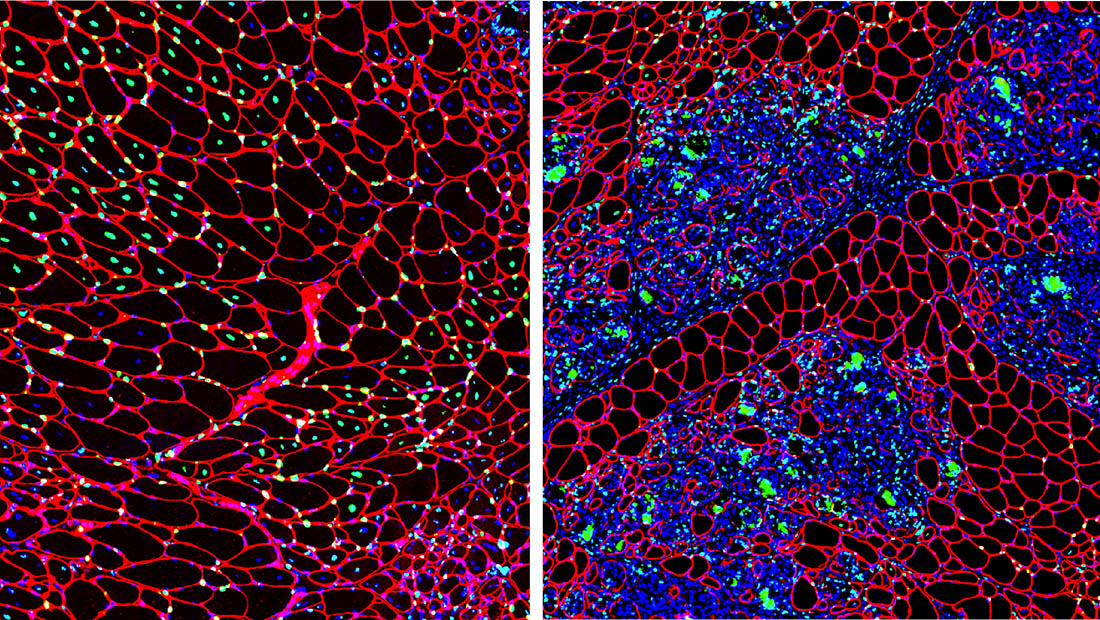
Despite the tremendous therapeutic potential of gene therapy for Duchenne and other muscular dystrophies, a major clinical challenge has been achieving efficient and targeted drug delivery and retention with the skeletal muscle and heart to improve drug efficacy and avoid off-target complications. Recently, we discovered the mechanism of PMO-based antisense oligonucleotide (AO) delivery to dystrophic muscle and found a prominent role for macrophages in the targeted delivery of AO to myofibers. We found that AO is readily sequestered within infiltrating macrophages that naturally target the damaged muscle, allowing for prolonged AO bioavailability within the muscle and efficient AO delivery to satellite cells and myofibers. Importantly, we showed that AO persists within infiltrating macrophages for days after it has been eliminated from circulation, indicating that they serve as a persistent drug reservoir within inflamed muscle, dramatically extending drug pharmacokinetics. These findings point to a potentially novel strategy for improving body-wide AO delivery in the context of disease pathogenesis, involving the use of inflammatory cells as a Trojan horse to improve the targeted delivery and bioavailability of these promising therapies. Currently, our lab is investigating the use of macrophages as cell-based delivery vehicles for efficient gene therapy delivery to dystrophic muscle to safely improve long-term pharmacokinetics and efficacy in the context of disease pathogenesis.
Skeletal Muscle and Cardiac Pathogenesis in Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is characterized by persistent skeletal muscle degeneration and chronic inflammation, which ultimately leads to progressive skeletal muscle loss and fibrosis. Further, cardiac function in DMD patients progressively worsens due to increased cardiomyocyte death, atrophy and fibrosis, which ultimately leads to heart dysfunction and potential failure. Recently, we have characterized the onset and progression of this disease in models that depict a range of pathological features of the human disease. We have demonstrated that skeletal muscle degeneration in DMD models involves altered interaction between inflammatory macrophages and fibro-adipogenic progenitors (FAPs), where elevated TGFβ activity contributes to the expansion of FAPs in muscle leading to fibrosis, chronic muscle loss, and impaired muscle satellite (stem) cell function. Currently, our work aims to investigate the complex cellular interactions and dynamic signaling processes during disease onset and progression through transcriptomic and proteomic profiling. The ultimate goal is to develop and test therapeutic strategies to inhibit these secondary pathologies that contribute to skeletal and cardiac muscle loss and fibrosis to improve the prognosis for DMD patients.